Cleaning Method
Cleaning is a complex process, the success of which is influenced significantly by several factors:
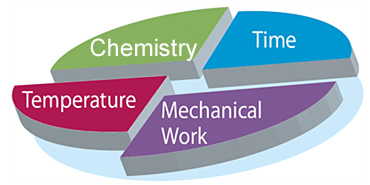
Chemistry
The chemistry, in this case meaning the cleaning agents used, helps in the decomposition of dirt components and thus simplifies the removal of dirt from surfaces. The detergents contained in cleaning agents improve the wetting of the item to be cleaned and the dirt carrying capacity of the water. This means that it is possible to prevent new deposits of dirt on the surfaces to be cleaned.
The selection of the cleaning agent to be used is focused on the dirt to be removed, the material of the items to be cleaned and the quality of water used for automated cleaning.
Temperature
High temperatures can accelerate physical, chemical and enzymatic processes and thus benefit removal of contaminations. Temperatures which are too high can, however, reduce enzyme activity. Another disadvantage of excessive temperature is the conversion carbonic acid contained in the water to carbonate via hydrogen carbonate which is then precipitated as a poorly soluble compound with the water hardening salts magnesium and calcium. This results in stubborn ‘lime deposits’.
Time
An extended reaction time for the cleaning solution makes the removal of dirt from the surface easier due to better swelling of the contaminants and a stronger chemical decomposition of the dirt components.
Mechanics
The use of mechanical agents such as the use of brushes or pressurized water jets enables bonding forces between dirt and surface to be overcome such that dirt can then be removed more easily.
Chemistry, temperature, time and mechanics are the four components of the so-called Sinner’s Circle which describes the mode of action of the cleaning process and which was named after the chemist Dr. Herbert Sinner: